Batteries are essential devices that store and convert chemical energy into electrical energy, powering a wide range of applications such as portable electronics, electric vehicles, power tools, and renewable energy systems. They can be classified into different types based on factors like size, voltage, chemistry, and rechargeability, playing a critical role in Power and Energy solutions.
The most common types of batteries include alkaline, nickel metal hydride (NiMH), and lithium-ion batteries. Alkaline batteries are inexpensive and disposable, offering a practical option for low-power applications, but they have a low energy density and are non-rechargeable. NiMH batteries, on the other hand, are rechargeable with a higher energy density than alkaline batteries, though they suffer from memory effect and self-discharge. Lithium-ion batteries represent the most advanced rechargeable option, delivering high energy density, long cycle life, and low self-discharge. However, they are more expensive and require careful handling to avoid issues like overheating or overcharging.
Beyond these, there are other specialized types of batteries designed for specific applications. Lead-acid batteries, for instance, are affordable and reliable but are heavy and contain toxic materials. Zinc-air batteries offer lightweight construction and high energy density but are sensitive to humidity and oxygen exposure. Molten salt batteries are ideal for large-scale energy storage applications due to their capacity but require high operating temperatures and complex management systems.
Understanding the types of batteries is crucial for selecting the right power source for different needs. Additionally, their integration with other technologies like types of LED drivers, types of electric motors, and types of battery chargers highlights their indispensable role in modern power and energy systems.
How Batteries Work
Batteries are devices that store chemical energy and convert it to electrical energy. They are used to power many devices, such as phones, laptops, flashlights, and cars. But how do they work?
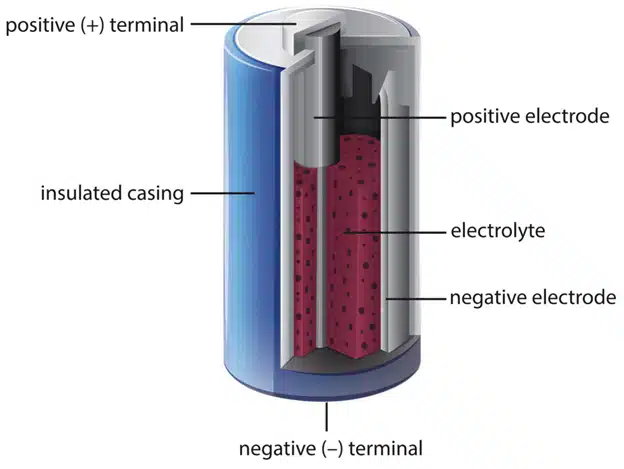
A battery consists of two electrical terminals called the cathode and the anode, separated by a chemical material called an electrolyte. The cathode is the positive terminal and the anode is the negative terminal. The electrolyte is a substance that can conduct electricity by allowing ions (charged atoms or molecules) to move between the terminals.
When a battery is connected to an external circuit, such as a light bulb, a chemical reaction takes place inside the battery. The anode releases electrons (negative charges) and the cathode accepts electrons. The electrons flow through the circuit, providing an electric current that can power the device. Meanwhile, the ions flow through the electrolyte, balancing the charge in the battery.
The chemical reaction in a battery depends on the type of materials used for the terminals and the electrolyte. Different types of batteries have different voltages (the amount of energy per unit of charge) and capacities (the amount of charge they can store). For example, alkaline batteries use zinc and manganese oxide as the terminals and potassium hydroxide as the electrolyte. They have a voltage of 1.5 volts and a capacity of about 2 ampere-hours (the amount of current they can provide for one hour). Lithium-ion batteries use lithium and cobalt oxide as the terminals and a liquid organic compound as the electrolyte. They have a voltage of 3.7 volts and a capacity of about 2.5 ampere-hours.
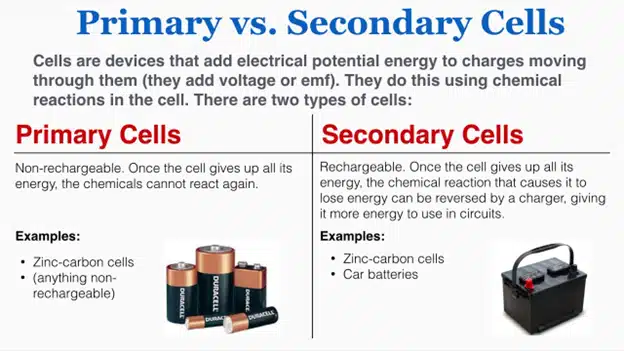
Some batteries are primary, which means they cannot be recharged once they are used up. They have to be replaced or recycled. Some batteries are secondary, which means they can be recharged by reversing the chemical reaction with an external power source. They can be used multiple times, which is more efficient and environmentally friendly.
Different Types of Batteries and Their Applications
Batteries are devices that store electrical energy and convert it into direct current (DC) electricity when needed. They are essential for powering many devices, such as laptops, smartphones, electric vehicles, and more. But not all batteries are the same. There are different types of batteries that have different characteristics, advantages, and disadvantages. In this blog post, we will explore some of the most common types of batteries and their applications.
Primary and Secondary Batteries
Primary and secondary batteries are two types of batteries that differ in their ability to be recharged. A battery is a device that converts chemical energy into electrical energy by using a chemical reaction between two electrodes and an electrolyte.
A primary battery is a battery that is designed to be used once and discarded. It cannot be recharged because the chemical reaction that produces electricity is irreversible, meaning that the electrodes and the electrolyte are consumed or transformed during discharge. Primary batteries have a high energy density and a long shelf life, but they are more expensive and less environmentally friendly than secondary batteries. Primary batteries are used for low-power devices that require infrequent or intermittent use, such as remote controls, flashlights, clocks, and smoke detectors. Examples of primary batteries are zinc-carbon, alkaline, lithium, and zinc-air batteries.
A secondary battery is a battery that can be recharged and used multiple times. It can be recharged because the chemical reaction that produces electricity is reversible, meaning that the electrodes and the electrolyte can be restored to their original state by applying an external electric current. Secondary batteries have a lower energy density and a shorter shelf life than primary batteries, but they are more economical and more environmentally friendly than primary batteries. Secondary batteries are used for high-power devices that require frequent or continuous use, such as phones, laptops, cameras, electric vehicles, and grid-scale energy storage systems. Examples of secondary batteries are lead-acid, nickel-cadmium, nickel-metal hydride, and lithium-ion batteries.
Alkaline Batteries
Alkaline batteries are a type of non-rechargeable batteries that use zinc and manganese dioxide as electrodes and an alkaline electrolyte, usually potassium hydroxide. They are also called alkaline-manganese batteries or LR batteries. They are widely used for high-power devices, such as toys, flashlights, cameras, and radios.
An alkaline battery consists of one or more cells, each with a negative terminal (anode) made of zinc powder and a positive terminal (cathode) made of a mixture of manganese dioxide and carbon. The terminals are separated by a separator that prevents short circuits but allows the electrolyte to flow through. The electrolyte is a substance that can conduct electricity by allowing ions to move between the terminals.
When an alkaline battery is connected to an external circuit, such as a flashlight, a chemical reaction takes place inside the battery. The anode releases electrons and zinc ions, and the cathode accepts electrons and hydrogen ions. The electrons flow through the circuit, providing an electric current that can power the device. Meanwhile, the zinc ions react with the hydroxide ions in the electrolyte to form zinc oxide and water.
The chemical reaction in an alkaline battery is irreversible, which means the battery cannot be recharged and must be replaced when it is used up. The typical voltage of an alkaline cell is 1.5 volts, which remains relatively constant until the end of discharge.
Alkaline batteries have some advantages and disadvantages compared to other types of batteries. Some of the advantages are:
- They have high energy density and capacity compared to other primary batteries.
- They have a long shelf life and low self-discharge rate.
- They have good performance at high currents and low temperatures.
- They are cheap and widely available.
Some of the disadvantages are:
- They are prone to leaks and corrosion if stored improperly or used beyond their expiry date.
- They contain toxic and corrosive materials, which are harmful to the environment and human health.
- They cannot be recharged or reused.
Alkaline batteries are used in various applications, such as toys, flashlights, cameras, radios, clocks, smoke detectors, and remote controls. However, they are being replaced by newer types of primary batteries, such as lithium and zinc-air batteries, which offer higher capacity, lower environmental impact, and longer lifespan.

Zinc-Carbon Batteries
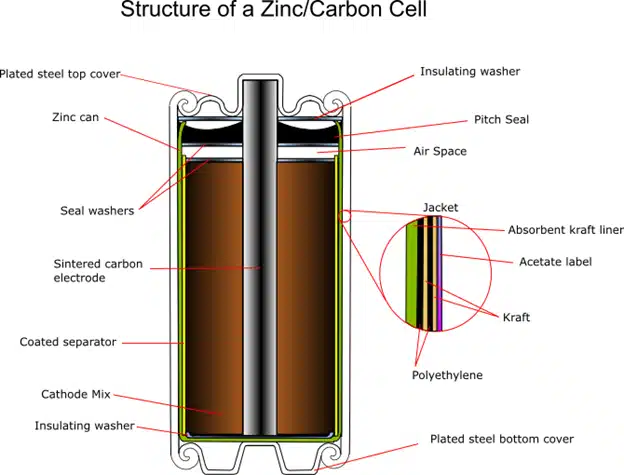
Zinc-carbon batteries are a type of non-rechargeable batteries that use zinc and manganese dioxide as electrodes and an ammonium chloride or zinc chloride electrolyte. They are also called carbon-zinc batteries or dry cell batteries. They are one of the earliest types of commercially available batteries and are widely used for low-power devices, such as remote controls, flashlights, clocks, and radios.
A zinc-carbon battery consists of one or more cells, each with a negative terminal (anode) made of zinc metal and a positive terminal (cathode) made of a mixture of manganese dioxide and carbon. The terminals are separated by a moist paste of ammonium chloride or zinc chloride. The electrolyte is a substance that can conduct electricity by allowing ions to move between the terminals.
When a zinc-carbon battery is connected to an external circuit, such as a flashlight, a chemical reaction takes place inside the battery. The anode releases electrons and zinc ions, and the cathode accepts electrons and hydrogen ions. The electrons flow through the circuit, providing an electric current that can power the device. Meanwhile, the ions flow through the electrolyte and the paste, balancing the charge in the battery.
The chemical reaction in a zinc-carbon battery is irreversible, which means the battery cannot be recharged and must be replaced when it is used up. The typical voltage of a zinc-carbon cell is 1.5 volts, which decreases gradually during discharge.
Zinc-carbon batteries have some advantages and disadvantages compared to other types of batteries. Some of the advantages are:
- They are cheap and widely available.
- They are simple and reliable.
- They can function in any orientation.
Some of the disadvantages are:
- They have low energy density and capacity compared to other primary batteries.
- They have high internal resistance and self-discharge rate.
- They contain toxic and corrosive materials, which are harmful to the environment and human health.
Zinc-carbon batteries are used in various applications, such as remote controls, flashlights, clocks, radios, toys, and smoke detectors. However, they are being replaced by newer types of primary batteries, such as alkaline and lithium batteries, which offer higher capacity, lower environmental impact, and longer shelf life.
Lithium Batteries
Lithium batteries are a type of rechargeable batteries that use lithium ions to store energy by creating an electrical potential difference between the negative and positive poles of the battery. They are widely used for portable devices, electric vehicles, and grid-scale energy storage systems.
A lithium battery consists of one or more cells, each with a negative electrode (anode) and a positive electrode (cathode) separated by an insulating layer called a separator. The separator blocks the electrons but allows the lithium ions to pass through. The electrodes are immersed in an electrolytic solution of lithium salts dissolved in organic solvents. The electrolyte is a substance that can conduct electricity by allowing ions to move between the electrodes.
When a lithium battery is connected to an external circuit, such as a phone, a chemical reaction takes place inside the battery. The anode releases electrons and lithium ions, and the cathode accepts electrons and lithium ions. The electrons flow through the circuit, providing an electric current that can power the device. Meanwhile, the lithium ions flow through the electrolyte and the separator, balancing the charge in the battery.
The chemical reaction in a lithium battery can be reversed by applying an external power source, such as a charger. This allows the battery to be recharged and used again. The typical voltage of a lithium cell varies depending on the type of materials used for the electrodes, but it is usually between 3 and 4 volts.
Lithium batteries have some advantages and disadvantages compared to other types of batteries. Some of the advantages are:
- They have high energy density and capacity compared to other rechargeable batteries.
- They have long cycle life and low self-discharge rate.
- They have fast charging and discharging rates.
- They have low maintenance and no memory effect.
Some of the disadvantages are:
- They are expensive and scarce compared to other rechargeable batteries.
- They are sensitive to high temperatures and overcharging or over discharging, which can cause thermal runaway or fire.
- They contain toxic and flammable materials, which are harmful to the environment and human health.
Lithium batteries are used in various applications, such as phones, laptops, cameras, drones, electric cars, electric bikes, solar panels, wind turbines, and backup power supplies. However, they also pose some challenges to recycling, safety, and sustainability.

Silver Oxide Batteries
Silver oxide batteries are a type of non-rechargeable batteries that use silver oxide and zinc as electrodes and an alkaline electrolyte. They are also called silver-zinc batteries or button cells. They are widely used for watches, hearing aids, calculators, and other small devices.
A silver oxide battery consists of one or more cells, each with a positive terminal (cathode) made of silver oxide and a negative terminal (anode) made of zinc. The terminals are separated by an electrolytic solution of potassium hydroxide or sodium hydroxide. The electrolyte is a substance that can conduct electricity by allowing ions to move between the terminals.
When a silver oxide battery is connected to an external circuit, such as a watch, a chemical reaction takes place inside the battery. The anode releases electrons and the cathode accepts electrons. The electrons flow through the circuit, providing an electric current that can power the device. Meanwhile, the ions flow through the electrolyte, changing the concentration of the solution.
The chemical reaction in a silver oxide battery is irreversible, which means the battery cannot be recharged and must be replaced when it is used up. The typical voltage of a silver oxide cell is 1.55 volts, which remains relatively constant until the end of discharge.
Silver oxide batteries have some advantages and disadvantages compared to other types of batteries. Some of the advantages are:
- They have a long shelf life of up to 10 years.
- They have high energy density and capacity compared to other primary batteries.
- They have steady voltage output.
- They have good performance at low temperatures and high currents.
Some of the disadvantages are:
- They are expensive and hard to find compared to other primary batteries.
- They contain toxic silver, which is harmful to the environment and human health.
Silver oxide batteries are used in various applications, such as watches, hearing aids, cameras, calculators, and metal detectors. However, they are now banned or restricted in some countries due to environmental concerns.

Mercury Batteries
Mercury batteries are a type of non-rechargeable batteries that use mercuric oxide and zinc as electrodes and an alkaline electrolyte. They were invented by Samuel Ruben in 1942 and are also called mercury cell, button cell, or Ruben-Mallory batteries.
A mercury battery consists of one or more cells, each with a positive terminal (cathode) made of mercuric oxide and a negative terminal (anode) made of zinc. The terminals are separated by an electrolytic solution of potassium hydroxide or sodium hydroxide. The electrolyte is a substance that can conduct electricity by allowing ions to move between the terminals.
When a mercury battery is connected to an external circuit, such as a watch, a chemical reaction takes place inside the battery. The anode releases electrons and the cathode accepts electrons. The electrons flow through the circuit, providing an electric current that can power the device. Meanwhile, the ions flow through the electrolyte, changing the concentration of the solution.
The chemical reaction in a mercury battery is irreversible, which means the battery cannot be recharged and must be replaced when it is used up. The typical voltage of a mercury cell is 1.35 volts, which remains practically constant until the end of discharge.
Mercury batteries have some advantages and disadvantages compared to other types of batteries. Some of the advantages are:
- They have a long shelf life of up to 10 years.
- They have steady voltage output.
- They have high energy density and capacity compared to other primary batteries.
- They have good performance at low temperatures and high currents.
Some of the disadvantages are:
- They are expensive and hard to find compared to other primary batteries.
- They suffer from memory effects, which means they lose capacity if they are not fully discharged before storage.
- They contain toxic mercury, which is harmful to the environment and human health.
Mercury batteries were used in various applications, such as watches, hearing aids, cameras, calculators, and metal detectors. However, they are now banned or restricted in many countries due to environmental concerns. In 1996, the Mercury-Containing and Rechargeable Battery Management Act was passed in the United States, which phased out mercury batteries and promoted recycling of other types of batteries.

Lead-Acid Batteries
Lead-acid batteries are a type of rechargeable batteries that use lead and lead oxide as electrodes and sulfuric acid as electrolyte. They were invented by Gaston Planté in 1859 and are the first type of rechargeable battery ever created. They are widely used for starter motors in vehicles, backup power supplies, and energy storage systems.
A lead-acid battery consists of one or more cells, each with a positive terminal (cathode) made of lead oxide and a negative terminal (anode) made of spongy or porous lead. The terminals are immersed in an electrolytic solution of sulfuric acid and water. The electrolyte is a substance that can conduct electricity by allowing ions to move between the terminals.
When a lead-acid battery is connected to an external circuit, such as a car engine, a chemical reaction takes place inside the battery. The anode releases electrons and the cathode accepts electrons. The electrons flow through the circuit, providing an electric current that can power the device. Meanwhile, the ions flow through the electrolyte, changing the concentration of sulfuric acid.
The chemical reaction in a lead-acid battery can be reversed by applying an external power source, such as a charger. This allows the battery to be recharged and used again. The typical voltage of a lead-acid cell is 2.1 volts, which decreases gradually during discharge.
Lead-acid batteries have some advantages and disadvantages compared to other types of batteries. Some of the advantages are:
- They have low cost and high availability.
- They have high power and current output.
- They have good performance at low temperatures.
- They have simple design and maintenance.
Some of the disadvantages are:
- They have low energy density and capacity compared to other rechargeable batteries.
- They suffer from sulfation, which means they lose capacity if they are not fully charged regularly.
- They have a high self-discharge rate, which means they lose charge when not in use.
- They contain toxic lead and sulfuric acid, which are harmful to the environment and human health.
Lead-acid batteries are used in various applications, such as cars, trucks, motorcycles, boats, golf carts, forklifts, solar panels, wind turbines, and emergency lighting. However, they are being replaced by newer types of rechargeable batteries, such as lithium-ion and nickel-metal hydride batteries, which offer higher capacity, lower environmental impact, and longer lifespan.

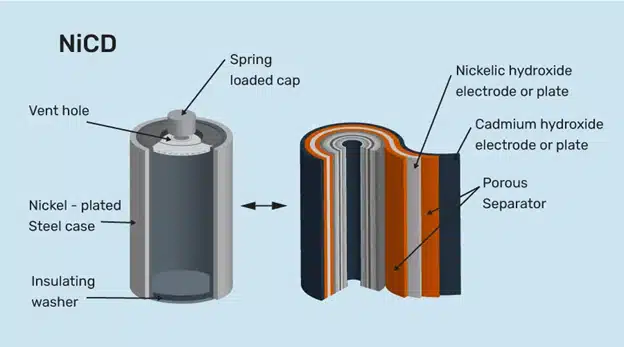
Nickel-Cadmium Batteries
Nickel-cadmium batteries are a type of rechargeable batteries that use nickel oxide hydroxide and metallic cadmium as electrodes. They were invented by Waldemar Jungner in 1899 and patented by Thomas Edison in 1902. They are also known as Ni-Cd or NiCad batteries.
A nickel-cadmium battery consists of one or more cells, each with a positive terminal (cathode) made of nickel oxide hydroxide and a negative terminal (anode) made of metallic cadmium. The terminals are separated by an electrolyte, which is a substance that can conduct electricity by allowing ions to move between the terminals. The electrolyte is usually a solution of potassium hydroxide.
When a nickel-cadmium battery is connected to an external circuit, such as a light bulb, a chemical reaction takes place inside the battery. The anode releases electrons and the cathode accepts electrons. The electrons flow through the circuit, providing an electric current that can power the device. Meanwhile, the ions flow through the electrolyte, balancing the charge in the battery.
The chemical reaction in a nickel-cadmium battery can be reversed by applying an external power source, such as a charger. This allows the battery to be recharged and used again. The typical voltage of a nickel-cadmium cell is 1.2 volts, which decreases little until nearly the end of discharge.
Nickel-cadmium batteries have some advantages and disadvantages compared to other types of batteries. Some of the advantages are:
- They have good cycle life and performance at low temperatures.
- They can deliver high current and power at fast discharge rates.
- They are relatively cheap and widely available.
Some of the disadvantages are:
- They have low energy density and capacity compared to other rechargeable batteries.
- They suffer from memory effects, which means they lose capacity if they are not fully discharged before recharging.
- They have a high self-discharge rate, which means they lose charge when not in use.
- They contain toxic cadmium, which is harmful to the environment and human health.
Nickel-cadmium batteries are used in various applications, such as portable power tools, photography equipment, emergency lighting, hobby RC, and medical devices. However, they are being replaced by newer types of rechargeable batteries, such as nickel-metal hydride and lithium-ion batteries, which offer higher capacity, lower environmental impact, and lower cost.
Conclusion
In conclusion, batteries come in many different types, each with its unique characteristics, advantages, and drawbacks. As technology evolves, new types of batteries are emerging to meet the ever-growing demand for energy storage solutions. Whether you’re looking for a portable option for outdoor activities or a reliable solution for larger applications, understanding the different types of batteries can help you make an informed decision. While some batteries may be more cost-effective, others may offer better performance and longer lifespan. By considering the specific requirements of your application, you can choose the best battery type for your needs and enjoy the benefits of reliable, sustainable energy storage.
FAQs about Different Types of Batteries
- What are some of the different types of batteries?
There are several types of batteries, including lead-acid, nickel-cadmium (Ni-Cad), nickel-metal hydride (Ni-MH), lithium-ion (Li-ion), and zinc-air. Each type has its own strengths and weaknesses, and the choice of battery depends on the specific application.
- What is the difference between a rechargeable and a non-rechargeable battery?
A rechargeable battery, also known as a secondary cell, is a battery that can be recharged and used multiple times. Non-rechargeable batteries, also known as primary cells or primary batteries, are disposable and cannot be recharged.
- What determines the lifespan of a battery?
The lifespan of a battery depends on several factors, including the type of battery, the climate and environment, the usage pattern, and the maintenance. For example, a rechargeable Ni-MH battery may have a lifespan of up to 1000 charge cycles, while a lead-acid battery may have a lifespan of up to 5 years.
- What is the self-discharge rate of a battery?
Self-discharge rate refers to the amount of power stored in a battery that is lost over time without being depleted by an external load. A high self-discharge rate means that the battery will discharge quickly even if it is not in use, which can be a problem in long-term storage and for applications that require infrequent use.
- What affects the performance of a battery?
The performance of a battery depends on several factors, including the type of battery, the state of charge, the temperature, the load, and the discharge rate. For example, the performance of a lithium-ion battery may decrease significantly at low temperatures, while lead-acid batteries may generate less power at high temperatures. It is important to choose the right battery for the specific application and to properly maintain it for optimal performance.



